what is the difference between aldehyde sugar and ketone sugar Aldehyde ketone difference between group functional aldehydes formula carbon structure common groups chemistry iv example form present
Ketones and aldehydes are both types of organic compounds that contain carbon, hydrogen, and oxygen atoms. They also belong to a group of chemicals called carbonyl compounds, which have a carbon double-bonded to an oxygen atom (C=O). Though they have similar structures, ketones and aldehydes exhibit different chemical properties that make them distinct from each other. Ketones are organic compounds that have a carbonyl group (C=O) with two alkyl or aryl groups attached to the carbonyl carbon. The simplest ketone is acetone, which has the chemical formula (CH₃)₂CO. Ketones are formed when an organic acid is oxidized and they often play crucial roles in biological processes such as metabolism. They are also used as solvents and in the manufacture of certain chemicals. On the other hand, aldehydes are organic compounds that have a carbonyl group (C=O) with one alkyl or aryl group and one hydrogen atom attached to the carbonyl carbon. The simplest aldehyde is formaldehyde, which has the chemical formula HCHO. Aldehydes are often found in natural products such as essential oils and are used in the production of plastics, resins, and other chemicals. They are also involved in several important biological processes, such as the oxidation of fats and carbohydrates. Despite being similar, ketones and aldehydes have several key differences. One of the most significant differences is their reactivity towards nucleophiles. Nucleophiles are molecules that are attracted to positively charged atoms, such as the carbon atom in the carbonyl group. Ketones are less reactive towards nucleophiles because the two alkyl or aryl groups attached to the carbonyl carbon create a steric hindrance that makes it difficult for nucleophiles to approach. Aldehydes, with only one alkyl or aryl group attached, are more susceptible to nucleophilic attacks. Another difference between ketones and aldehydes is the ease with which they can be oxidized. Ketones do not readily undergo oxidation reactions, while aldehydes can be easily oxidized to form carboxylic acids. This property makes aldehydes useful in several industrial processes, such as in the production of adhesives and detergents. In addition to their differences in chemical properties, ketones and aldehydes also have different physical properties. Ketones typically have higher boiling points than aldehydes due to their larger molecular weights and stronger intermolecular forces. Ketones are also less polar than aldehydes because the two alkyl or aryl groups attached to the carbonyl carbon create a partially positive charge that can offset the partially negative charge of the oxygen atom. In conclusion, while ketones and aldehydes share similar chemical structures, their chemical and physical properties make them distinct from each other. Ketones are less reactive towards nucleophiles than aldehydes, and they do not easily undergo oxidation reactions. Aldehydes, on the other hand, are more susceptible to nucleophilic attacks and readily undergo oxidation reactions. Understanding these differences is important for their industrial, biological, and medical applications.
If you are searching about Difference Between Aldehyde and Ketone – CueRead you’ve visit to the right web. We have 5 Pictures about Difference Between Aldehyde and Ketone – CueRead like Difference Between Ketone and Glucose | Difference Between, Difference Between Aldehyde and Ketone | Structure, Properties, Naming and also Difference Between Aldehyde and Ketone – CueRead. Here you go:
Difference Between Aldehyde And Ketone – CueRead
 www.cueread.comDifference Between Ketone And Glucose | Difference Between
www.cueread.comDifference Between Ketone And Glucose | Difference Between
 www.differencebetween.netglucose ketone summarized below
www.differencebetween.netglucose ketone summarized below
الفرق بين الألدهيد والكيتون - أخبار 2023
 ar.weblogographic.comDifference Between Aldehyde And Ketone | Structure, Properties, Naming
ar.weblogographic.comDifference Between Aldehyde And Ketone | Structure, Properties, Naming
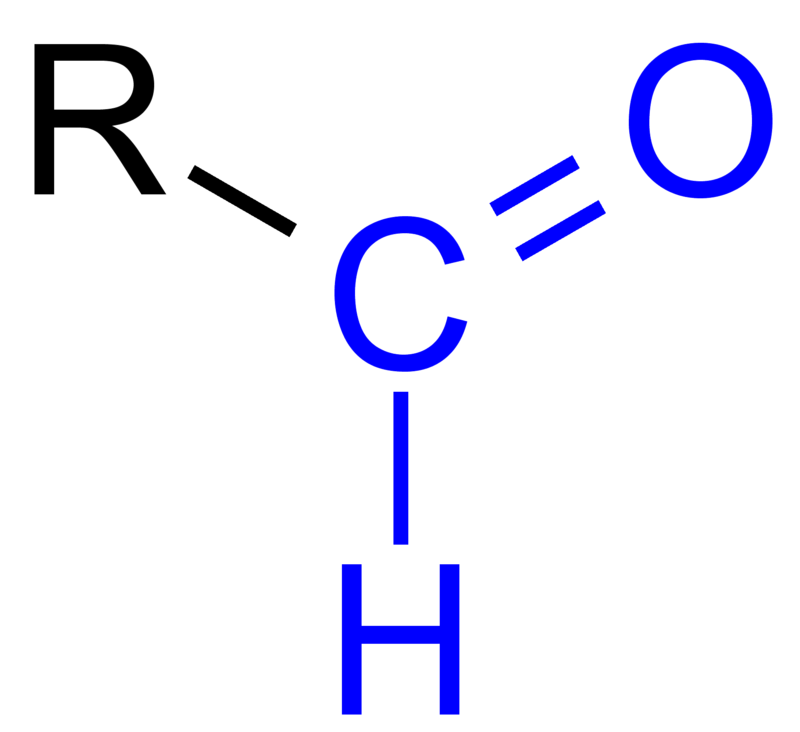 pediaa.comaldehyde ketone difference between group functional aldehydes formula carbon structure common groups chemistry iv example form present
pediaa.comaldehyde ketone difference between group functional aldehydes formula carbon structure common groups chemistry iv example form present
PPT - Aldehydes And Ketones PowerPoint Presentation, Free Download - ID
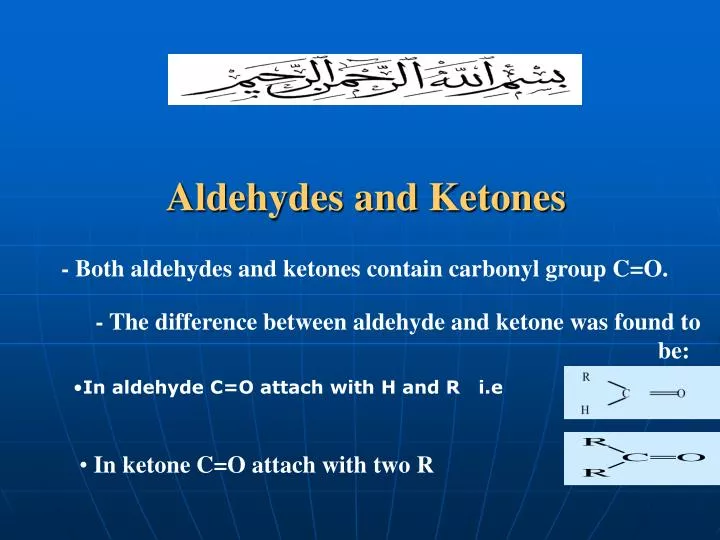 www.slideserve.comaldehydes ketones aldehyde ketone difference between ppt presentation carbonyl powerpoint
www.slideserve.comaldehydes ketones aldehyde ketone difference between ppt presentation carbonyl powerpoint
Difference between aldehyde and ketone. Difference between ketone and glucose. Glucose ketone summarized below